Hug & Barrett versus IPCC
by
Heinz Hug and Jack Barrett
(For an `Open Review' of this paper, click here)
![]()
The IPCC mechanism for global warming relies entirely upon the theory of radiative transfer. This assumes a long-term radiative equilibrium for the interaction between incoming solar radiation and out-going long wavelength radiation. The theory is correct for ‘blue skies’ and has to be severely modified to take into account the effects of clouds and the transfer of energy by non-radiative processes to the troposphere of latent heat of evaporation of water. In particular, the IPCC mechanism is based on the re-emission of absorbed terrestrial radiation, some of which is directed downwards and causes the extra warming of the Earth’s surface. Hug and Barrett agree that extra carbon dioxide in the atmosphere will further impede the flow of radiation from Earth to space. They argue that the extra carbon dioxide will also further impede the radiation returning to Earth from where the atmosphere has absorbed it and that this factor is not included in the IPCC mechanism. Hug and Barrett argue that the energy budget indicates that the warming of the lower atmosphere is by (i) heat transfer, (ii) latent heat transfer and (iii) radiation from the Earth’s surface. The fractions of the heat flux producing atmospheric warming1 by the three processes are 19%, 61% and 20% respectively. The warming by radiative means of only 20% arises as the resultant emission of 350 W m-2 from the surface minus the 324 W m-2 returning to the surface from the atmosphere. [Of the 390 W m-2 emitted by a cavity radiator at 288 K, 40 W m-2 are not absorbed by the atmosphere and reach space through the infrared ‘window’.] The 20% warming by radiation is more consistent with the Hug and Barrett contention that the flux of 350 W m-2 absorbed by the lower atmosphere (by the molecules of water and carbon dioxide) is converted mainly into the translational energy of the constituent molecules, which thereby have an increased temperature. The increased temperature of the lowest part of the atmosphere causes the transfer of thermal energy to the higher parts by convection and the evaporation of water. Certainly the air at higher temperature will emit more radiation, but this seems to be of lower priority (20%) than the other two processes. The warming mechanism operates every morning around the Earth and it is clear to Hug and Barrett that at no time is there a true equilibrium in existence. Barrett2 has presented similar arguments previously in response to the obviously oversimplified explanation of the greenhouse effect presented by the IPCC3. His paper was criticised by Houghton4 and Shine5 who pointed out some errors. These were replied to by Barrett.6 Braterman7 published a further criticism which indicated that he did not understand the problem and his paper was refuted by Barrett8 and Courtney.9 The outcome of this argumentation is that additional CO2 is expected to increase global temperature, but the extent of the warming and the mechanism of the warming are still debatable, as outlined above.
The above proposed mechanism of terrestrial radiation being absorbed by the ‘greenhouse’ gases, producing rotationally and vibrationally excited states that are then mainly degraded to their ground states by the conversion of their excitation energy into the translational energy of colliding molecules of dinitrogen and dioxygen has been criticised as violating Kirchhoff’s law. This law specifies that a good absorber is a good emitter and the IPCC supporters have interpreted this to indicate that they should in equal measure emit the terrestrial radiation absorbed by the greenhouse gases. This is true only for a system in thermal equilibrium. The IPCC explains the phenomenon in terms of radiation and imply that molecules do not obtain kinetic energy (heat) by absorption of radiation by greenhouse gases. The IPCC state that the air is warmed by contact with the warmed soil/ocean surfaces. The up-streaming of the atmosphere is caused by such warming as are winds, storms, hurricanes and tornadoes. The IPCC assumes that photons are recycled by CO2 causing 90% of the absorbed radiation to return radiatively to the surface.1 Because the atmosphere is nearly completely opaque in the CO2 region the re-emitted radiation can only ‘escape’ through the open IR window to space. These ideas were confirmed to Hug at the DECHEMA colloquium. The misunderstanding arises from the IPCC regarding the processes operating under conditions of true equilibrium, where the Kirchhoff law operates, whereas under the non-equilibrium conditions occurring daily in any part of the globe at any time, the law is inapplicable. Under those daily circumstances, when warming of the atmosphere is occurring, the molecules of the atmosphere absorb more radiation than is emitted. As cooling takes place the reverse is the case, radiative gases emit more than they absorb, acquiring their excitation energy mainly by collisional processes.
Hug showed measured
spectra of (i) carbon dioxide, (ii) carbon dioxide and helium and (iii)
carbon dioxide with dinitrogen, where the sample cells contained the
same pressure of carbon dioxide and where the pressures of helium and
dinitrogen were both one atmosphere. The spectra are shown in Fig. 1
The three spectra differed because of the different pressure broadening of the vibration-rotation bands of the carbon dioxide in the three cases.10, 11 The integrated line strengths of the band spectra are given in Table 1. Table 1 Integrated line strengths of CO2 spectra
In case (i) there was self-broadening (resonance broadening) and in cases (ii) and (iii) the broadening depended upon the nature (mass) of the ‘foreign’ gas. The extent of broadening in all three cases was consistent with theory and showed that the absorbing molecules of carbon dioxide did experience collisional interactions that altered their spectral behaviour. The observations were consistent with the Hug and Barrett contention that radiation absorbed by carbon dioxide was most likely to be converted to the translational energy of colliding molecules. Dr Birk particularly, supported by Drs Bakan and Hollmann, stated that Hug’s observations were incorrect because of the relatively poor resolution of the spectrometer used and that, in any case, the effects of ‘foreign’ gases were not as observed. The effects were due to instrumental errors and that pressure broadening was a function of pressure only and not dependent upon the nature of the ‘foreign’ gas. They claimed that the spectra presented by Hug could be explained by deviations from the Beer-Lambert law and showed a figure of a similar case. Fig.2 shows spectra of methane in the presence of foreign gases.12 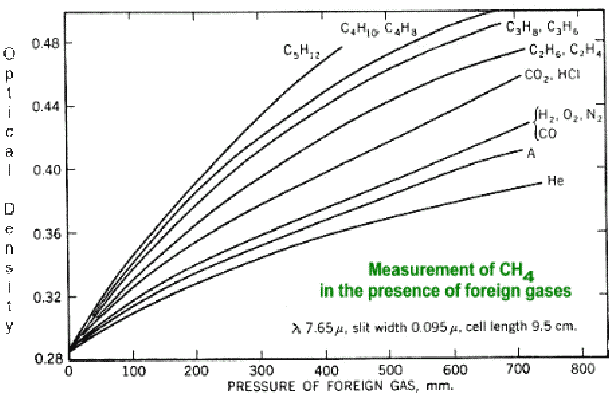
Hug and Barrett strongly disagree about this view of pressure broadening and maintain that Hug’s spectra show real effects. The resolving power of the spectrometer used by Hug was exactly the same for all three spectra, so although somewhat different observations would be obtained by using an instrument with a different resolving power, the effects of the ‘foreign’ gases would still be observed. It is not a Beer-Lambert law misinterpretation by Hug since his measurements were carried out at constant pressure (1 bar) and at constant temperature (22 ° C). The spectra shown by the IPCC people are measured at different pressures and are therefore not a proof of the allegation that Hug had used the Beer-Lambert law incorrectly. Note that in Fig.2 the optical density is given at a ‘peak’ wavelength of 7.65 m m and not as an integrated line strength over a wider range (as in Table 1, for instance). It has to be emphasized that in all three spectra the absorption of 1017 CO2 molecules cm-2 (entrance to the sample cell) were measured. Thus Hug’s spectra clearly show qualitatively that most of the infra-red radiation absorbed by greenhouse gases is converted to kinetic energy. This concerns mostly the main components of the atmosphere (N2 and O2). Therefore, the exclusive photon recycling process on which all climate modelling is based has to be rejected or modified. The IPCC people used another argument, saying that Hug’s spectra are affected by the effect of slit-width (effectively the ‘Fourier transform stop’) of the spectrometer used on the shape of the spectral bands. This argument is also flawed because Hug used the same spectrometer with the same parameters and thus any errors would be consistent and relatively the same. The differences in the integrated line strengths can only be explained in terms of the conversion of a fraction of the absorbed radiation to the kinetic energy of the molecules of the air.10
Atmospheric sensitivity Barrett gave figures for the sensitivity of the atmosphere in terms of the warming in degrees K that would be caused by an increase in atmospheric forcing of 1 W m-2. (i) The accepted extent of global warming is 33°K, this arises form a forcing of 235 W m-2 giving a value for the sensitivity of 0.14°K (W m-2)-1. This is essentially a crude value because the relationship between forcing and warming is not linear, but logarithmic. It would be expected that the sensitivity to any enhancement of forcing would be considerably less than this crude value. (ii) After the Mount Pinatubo eruption the Earth’s temperature decreased by 0.3°K and the estimated reduction in forcing was 2 W m-2. This gives a value of 0.15 (W m-2)-1 for the sensitivity. (iii) The Stefan-Boltzmann equation linking the energy of emission of a cavity radiator to its temperature: E = s T4 may be differentiated with respect to temperature: dE/dT = 4s T3 and inversion gives a value for the sensitivity: dT/dE = 1/4s T3 If a value of 288°K (a mean value for the troposphere at sea-level) is inserted into the equation the value for the sensitivity is 0.18 (W m-2)-1. The three values produced are remarkably similar, but vary greatly from the IPCC value obtained for the ‘business-as-usual’ scenario by theoretical calculations of the effects of doubling the carbon dioxide content of the atmosphere of a 2.5°K increase in temperature resulting from an increase in forcing of 3.8 W m-2 which gives the IPCC value for the sensitivity as 0.66 (W m-2)-1.
Spectral contributions from CO2 and H2O In their calculations of the effects on the atmosphere of a doubling of carbon dioxide, the IPCC carry out their line-by-line calculations and arrive at the value quoted above. They ignore completely the spectral contributions from the water molecules present in the atmosphere. Hug pointed out that the ‘wings’ of the carbon dioxide bands were too weak to explain the predicted effects by the IPCC, but the IPCC supporters claimed that there were no errors in the IPCC assumptions and calculations. Hug emphasized that at a height above the ground of 1000 m 97% of the carbon dioxide bands are saturated in the current atmosphere, i.e. transmission, T = 0. The IPCC claims that the absorption in these bands are responsible for a greenhouse effect of 7.2°C and claims that a further warming of 5.8°C will occur if the carbon dioxide level doubles. Hug expressed his disbelief in these results, but the IPCC supporters confirmed that the predicted warming would come to pass. Barrett showed the spectral effects on a 100 m thick layer of atmosphere containing 360 and 720 ppmv CO2 in the presence and absence of a pressure of water vapour equivalent to a relative humidity of 50%. The results are given in Table 2. Table 2 Results of HITRAN calculations
It is clear from the calculations that there is a great amount of overlap between the spectra of CO2 and H2O and that in the presence of water, the effect of doubling carbon dioxide is considerably reduced compared to the effect in the absence of water vapour. Hug and Barrett consider that the spectral overlap, ignored by the IPCC, is the reason for the sensitivity being exaggerated by the IPCC and that the real value is considerably less that that accepted by the IPCC. If Hug and Barrett are correct, the effects of doubling carbon dioxide in the atmosphere seem to be minimal and are no cause for alarm and the extensive alteration of national economies. The results presented by Barrett were relevant to absorption of terrestrial radiation as dealt with in the original paper3 that caused so much argument. They do not represent the radiation fluxes at the top of the 100 m path, these would contain contributions from the upward thermal radiation from that region of the atmosphere.
Water (cloud) feedback estimations All models of the atmosphere are in agreement that a doubling of carbon dioxide leads to some increase of the mean temperature, although the magnitude of the increase depends upon the particular model used. The increase in water vapour present in the warmed atmosphere leads to greater cloud cover and this alters the mean temperature, this is described as the water vapour feedback. The various models in use give very varied values for the water vapour feedback and do not agree on the sign of the value. If the MRI (Japan) model values are ignored, because they are clearly in error, the values for the feedback vary from the BMRC (Australia) figure of –1 W m-2 to the LMD (France) figure of +1.75 W m-2 as shown13 in Fig. 3.
BMRC = Bureau of Meteorology Research Center (Australia); NCAR = National Center for Atmospheric Research (USA); MGO = Main Geophysical Observatory (Russia); CSIRO = Commonwealth Scientific and Industrial Research Organization (Australia); MPI = Max Planck Institute for Meteorology (Germany); UKMO = Hadley Centre (UK); GFDL = Geophysical Fluid Dynamics Laboratory (USA); CCSR = Center for Climate System Research (Japan); LMD = Laboratoire de Météorologie Dynamique (France) The extent of this variation does not give much confidence in the current state of modelling, doubts which must extend to the modelling of the overall sensitivity and to the general prediction capability of models in general. Local thermodynamic equilibrium Local thermodynamic equilibrium is a concept that is misunderstood by some. Its normal interpretation is that the collision rate in the system is sufficiently high to ensure that the equipartition principle holds, i.e. that all available modes are populated according to the appropriate value of the Boltzmann factor. This does not mean that any part of the system is in thermodynamic equilibrium with its immediate surroundings. One cubic metre of the atmosphere may very well be in LTE and the adjoining six to fourteen cubic metres could also be in LTE, but it is not necessary for all the systems to be in true thermodynamic equilibrium with each other. If this were to be the case throughout the atmosphere, there would be true thermodynamic equilibrium and nothing would change. The atmosphere is not in true thermodynamic equilibrium and is constantly changing, although in the regions below 90 km altitude local thermodynamic equilibrium holds. Only above such altitudes does LTE break down as the natural lifetime of excited states of the greenhouse molecules are then longer than the rates of collisional excitation and the emission rates are governed by the rates of collision.
The impossibility of the doubling of atmospheric CO2
Conclusions
The authors would be grateful for any feedback and discussion on any of the points raised in the paper.
|
![]()
Return to `Climate Change Guest Papers' page
Return to `Still Waiting for Greenhouse' main page